RESTORE SKIN’S VITALITY DURING MENOPAUSE
Mystro Revive Renewing Serum addresses the visible skin changes associated with perimenopause and menopause such as dryness, dullness, loss of firmness, uneven texture, and the appearance of lines and wrinkles. Dermatologist tested on peri- and menopausal women, Mystro Revive Renewing Serum is formulated without parabens, fragrance and dyes, suitable for sensitive skin and non-comedogenic.

THE EFFECTS OF HORMONAL CHANGES ON SKIN HEALTH
Estrogen plays a vital role in maintaining skin health, influencing collagen and elastin synthesis, hydration, and overall skin vitality. At the onset of perimenopause and throughout menopause, a decrease in estrogen disrupts these crucial skin processes impacting skin balance and leading to a variety of noticeable skin changes.
Mystro Revive Renewing Serum rejuvenates the look of skin affected by hormonal decline associated with perimenopause and menopause. Powered by 2 proprietary biotechnologies, P.A.T.H.[13] and TAP, Mystro Revive Renewing Serum helps revitalize dull, dry skin and visibly improves the appearance of elasticity, lines and wrinkles, and skin tone and texture.
Mystro Revive Renewing Serum rejuvenates the look of skin affected by hormonal decline associated with perimenopause and menopause. Powered by 2 proprietary biotechnologies, P.A.T.H.[13] and TAP, Mystro Revive Renewing Serum helps revitalize dull, dry skin and visibly improves the appearance of elasticity, lines and wrinkles, and skin tone and texture.
.19?sw=750&sh=750&sm=cut)
Mystro Revive Renewing Serum
KEY BIOTECHNOLOGY INGREDIENTS

Formulated with proprietary P.A.T.H.[13] biotechnology to address the unique needs of skin affected by hormonal decline during perimenopause and menopause.
FEATURES & BENEFITS
- P.A.T.H.[13] biotechnology, a proprietary blend of 13 plant adaptogens, addresses the visible effects of hormonal changes on skin, helping to restore the skin’s natural balance.
- Fortified with TAP biotechnology to target intrinsic free radicals to mitigate oxidative stress.
- Dermatologist tested on perimenopausal and menopausal women to show improvements in elasticity, lines & wrinkles, and hydration with sustained improvements over 16 weeks.
OUTSTANDING CLINICAL RESULTS
PATIENTS REPORTED*:
- 90% skin looks plumper & revived
- 92% skin looks rejuvenated and feels soothed
- 96% overall quality of skin is improved
Significantly improved appearance of crepey skin, lines and wrinkles, and hydration.
- 32% IMPROVEMENT in the appearance of Crepey Skin after 16 weeks
- 22% IMPROVEMENT in the appearance of Lines & Wrinkles after 16 weeks
- 60% IMPROVEMENT in the appearance of Skin Hydration after 16 weeks
*A multi-center, dermatologist- and plastic surgeon-led clinical trial evaluated twice-daily use of Mystro Revive Renewing Serum in peri- and menopausal females +46 years of age, FST I-VI with fine-to-moderate lines/wrinkles and mild-to-moderate elastosis and crepey skin over 16 weeks. (N=53)
- Dispense 1-2 pumps and apply to face in the morning and evening. Massage thoroughly into skin including around hairline and eyebrows.
- Protect with sunbetter sunscreen during the day for additional skincare benefits.

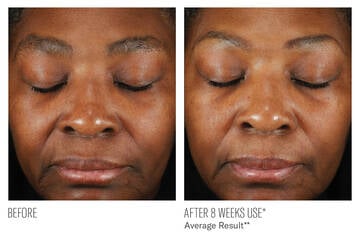
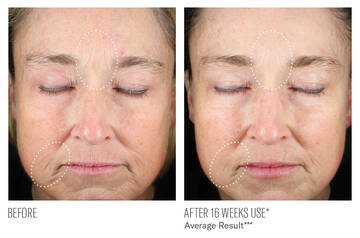
CLINICALLY DEMONSTRATED RESULTS
IMPROVEMENTS IN DULLNESS AND LINES/WRINKLES
***Results based on a 12-week double-blinded, randomized trial conducted on 52 subjects.
IMPROVEMENTS IN ELASTOSIS AND LINES/WRINKLES
*Unretouched clinical photography on clean, product-free skin.
**Image descriptions are based on average measurements of Dullness and Lines/Wrinkles assessed by the investigator from Baseline at 8-weeks of twice-daily use. (N=53)
***Image descriptions are based on average measurements of Elastosis and Lines/Wrinkles assessed by the investigator from Baseline at 16-weeks of twice-daily use. (N=53)
**Image descriptions are based on average measurements of Dullness and Lines/Wrinkles assessed by the investigator from Baseline at 8-weeks of twice-daily use. (N=53)
***Image descriptions are based on average measurements of Elastosis and Lines/Wrinkles assessed by the investigator from Baseline at 16-weeks of twice-daily use. (N=53)










%20(1).jpg?sw=270&sfrm=png&q=70)
.jpg?sw=270&sfrm=png&q=70)
.jpg?sw=270&sfrm=jpg&q=70)